Pharma Document Management System
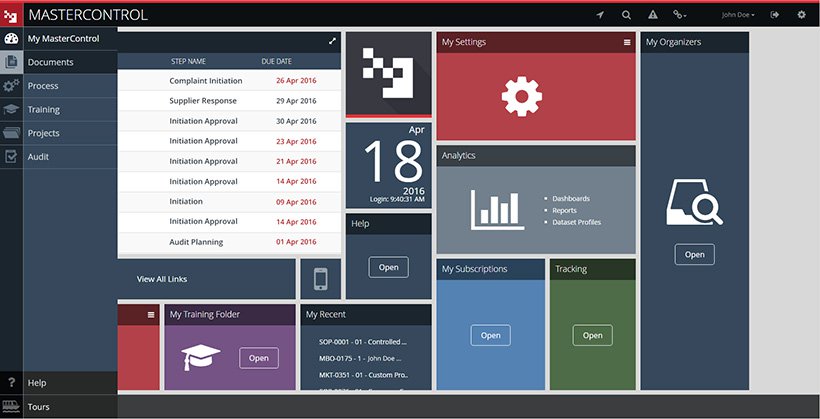
Pharma document management system. This document management software is for Startups SMEs Agencies Enterprises and can be deployed on Any. Document indexing is the process of associating or tagging documents with different search terms. MasterControl Documents is an advanced pharmaceutical document control software system that seamlessly integrates with the entire family of MasterControl quality management software solutions.
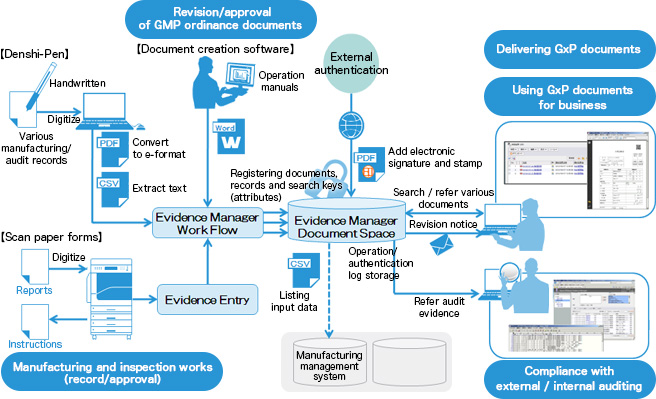
Applicable for Document Management system in Quality Assurance department Responsibility. AmpleLogic EDMS Software is a power-packed electronic document management system exclusively designed for Pharmaceutical and Biotech companies developed and implemented exclusively to meet the cGMP needs of Pharma domain with 21CFR Part 11 compliance EU Annex 11 compliance can easily eliminate all the challenges faced by different companies. Scanner EmailManual UploadBulk Upload etc.
Try Now for Free. The Pharma IT Cloud EDMSQMS is a complete GxP-validated Document Quality and Training Management system based on Ennov software and pre-configured according to industry best practices. Document Management System in Quality Assurance Department Objective.
Global Regulatory agencies expects that all regulated companies follow current. Cloud-based tools include document management training CAPA validation and more. DOCUMENT MANAGEMENT SYSTEM SOFTDMS.
MasterControls mission is to create software-as-a-service SaaS products which help pharma and life sciences organizations bring products to market faster with solutions for the entire pharma lifecycle. To lay down a procedure for Management of Documents in Quality Assurance Department. Standard Operating Procedure SOP Objective.
Crown Document Management is a document management system for Pharmaceuticals industry. Ad Get the Management Systems your competitors are already using - Start Now. Document Management System Law Firm.
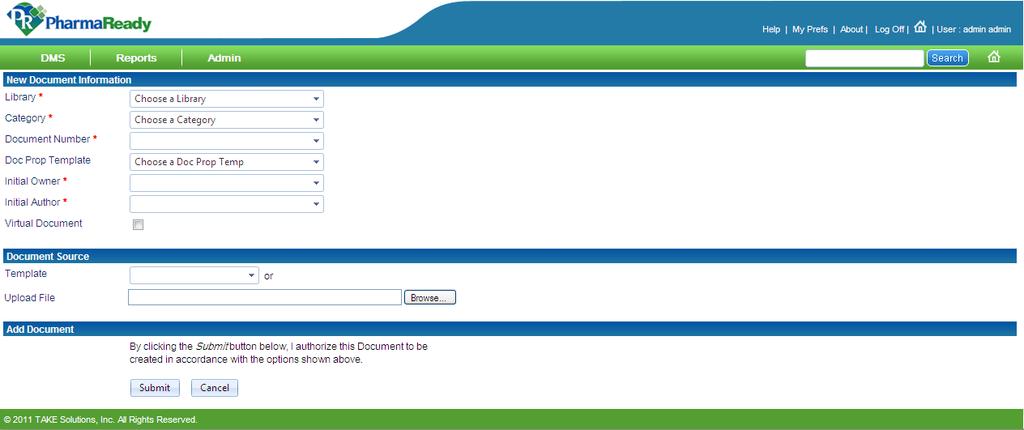
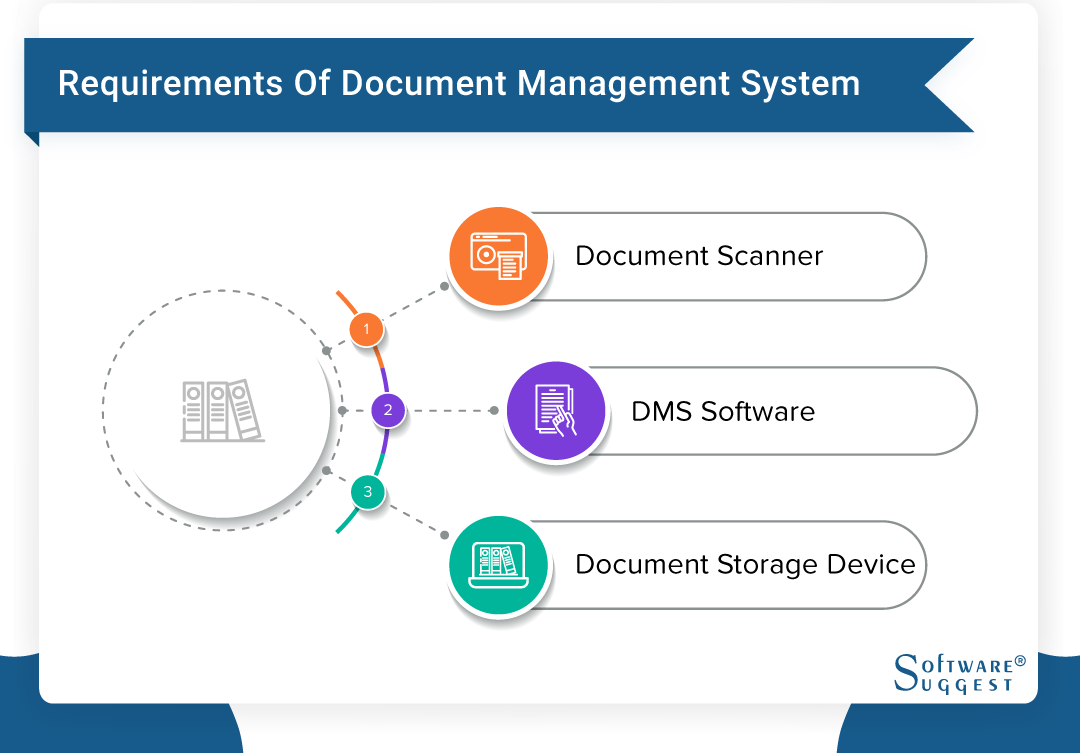
One unified end-to-end Document. The ideal Document Management System should allow inputting files trough the following sources.
It is a configurable and easy-to-use software solution that helps pharmaceutical companies attain and sustain cGMP compliance.
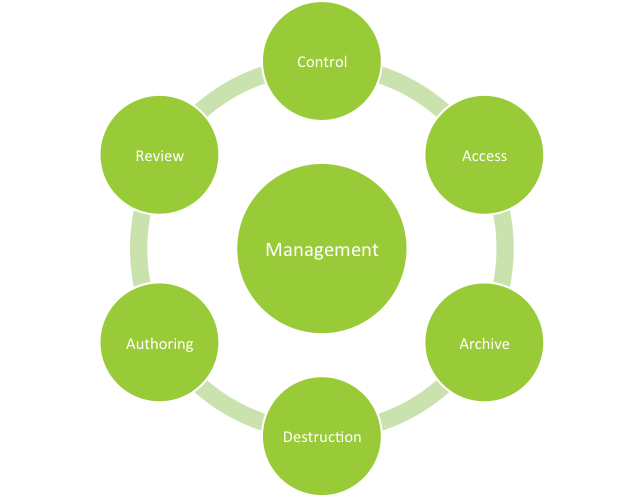
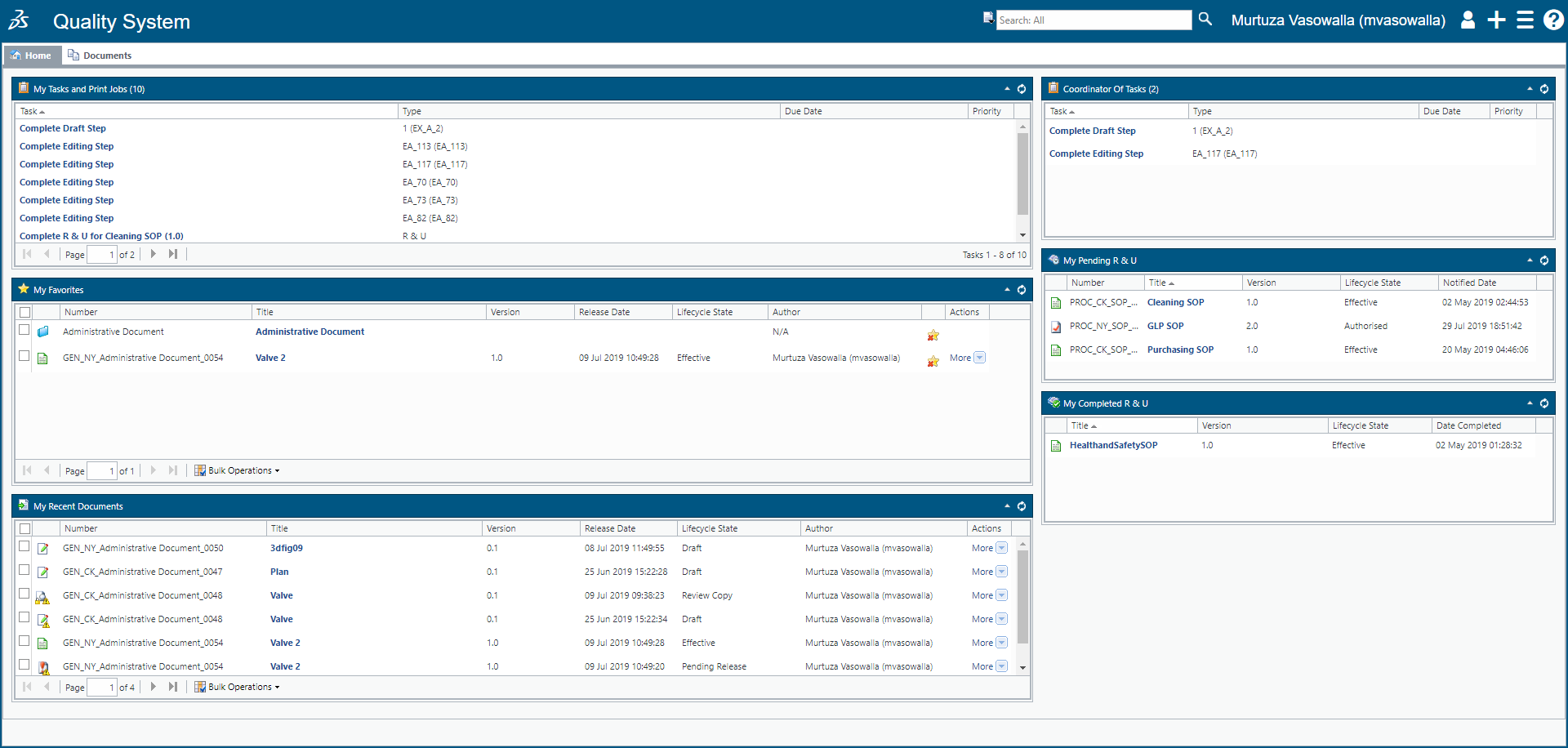
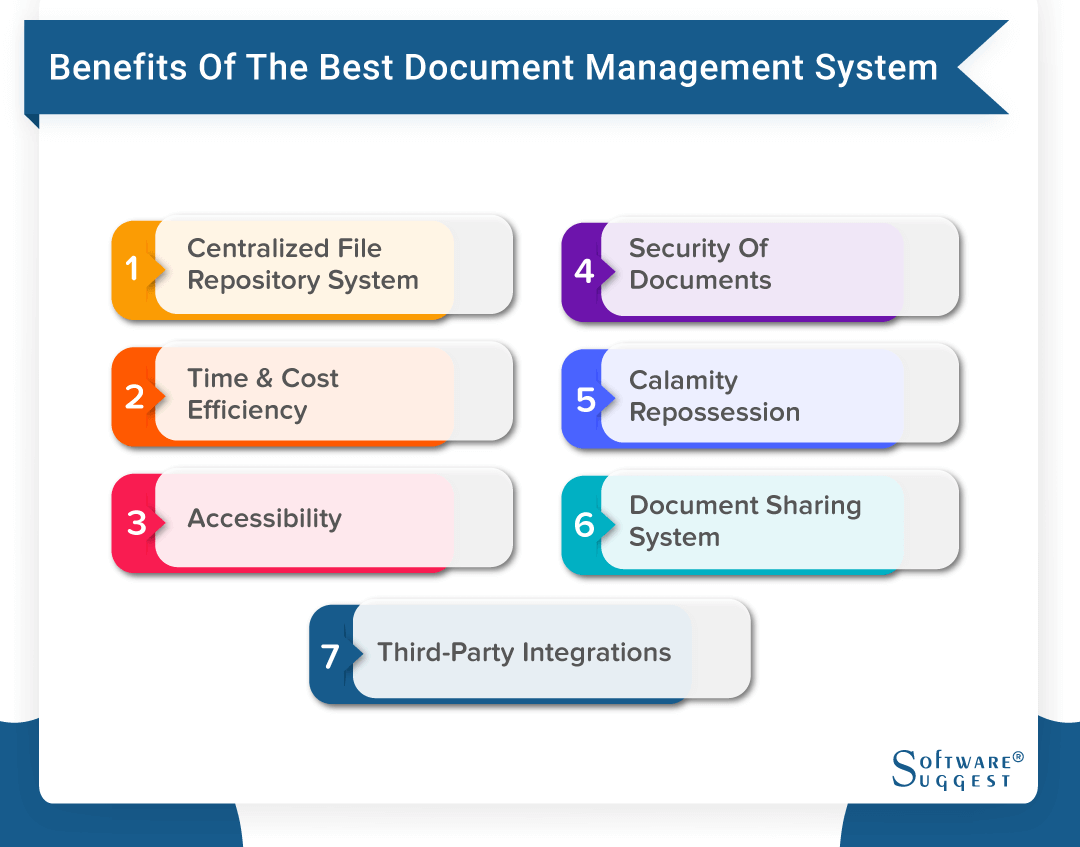
Choose Your Management Systems from the Premier Resource for Businesses. Upload Edit Sign PDF Documents Online. Prepare for risk management through essential activities critical to design and implementation of a risk management program. Addressing concerns an effective document control and management system is crucial to ensure documents are preserved traceable retrievable revisable and approvable when needed. Standard Operating Procedure SOP Objective. Categorize systems and information based on an impact analysis. MasterControls pharmaceutical document management system reduces document cycle time and simplifies document management by automating routing notification and follow-up escalation and approval of a pharmaceutical companys documents. One unified end-to-end Document. Document Management System in Quality Assurance Department.
SOP for Document Management System or Document and Data Control in Pharmaceuticals. Our cost-effective solution boasts zero upfront implementation costs and comes with the support of Pharma ITs expert consultants. Ad Get the Management Systems your competitors are already using - Start Now. Key features include Audit Trail Document Management Project Management and. Crown Document Management is a document management system for Pharmaceuticals industry. Prepare for risk management through essential activities critical to design and implementation of a risk management program. Head- QA shall be accountable for compliance of this SOP.






























Post a Comment for "Pharma Document Management System"